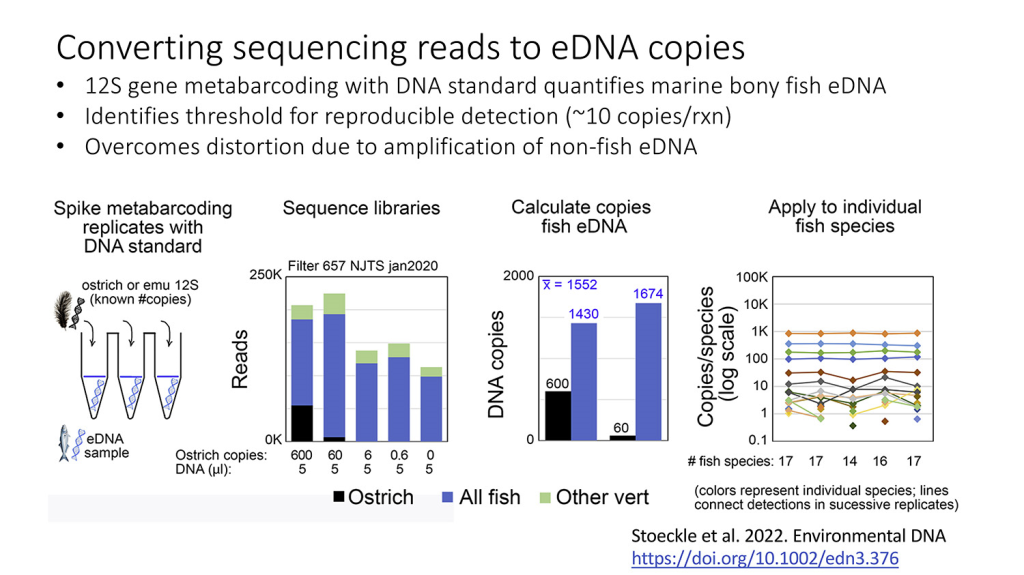
NOAA has posted the video of their ‘Omics Seminar Series: eDNA-Dominant Marine Fish Species Characterize Coastal Habitats presented on 28 February, 2024 by Mark Stoeckle and Jesse Ausubel. The 1-hour seminar is full of new results and ideas about using eDNA data to characterize marine regions and features Mark’s excellent graphics.
Title: eDNA-Dominant Marine Fish Species Characterize Coastal Habitats: an eDNA-Based Classifier Approach to Aid Marine Biogeography and Ocean Monitoring by Mark Stoeckle & Jesse Ausubel
Abstract: A small minority of species typically account for the great majority of individuals or biomass. Here we characterize marine coastal habitats based on abundance of marine fish environmental DNA. We designate the ten most eDNA-abundant fish species in each habitat as eDNA-dominant species. eDNA-dominant species are similar within but differ among habitats and seasons and accord with abundance by traditional survey methods. “Classifiers” based on eDNA-dominant fish species could help map marine fish habitats and monitor changing oceans. Advantages include relatively low sampling requirements, a single technology applicable to diverse habitats, and ease of application to multiple datasets.