 Just as DNA analysis regularly overturns seemingly solid eyewitness identifications in crime investigations, routine DNA analysis can also help biologists avoid blunders. In 28 August 2007 Mol Ecol, researchers from University of Colorado, New Mexico State University, Pisces Molecular, and Brigham Young University report that over 20 years of restocking efforts in western US aimed at restoring native populations of endangered greenback cutthroat trout Oncorhynchus clarkii stomias have mostly been restocking a non-native, non-endangered subspecies, Colorado River cutthroat trout O. c. pleuriticus. They trace the confusion to repeated introductions beginning in the late 1800s of Colorado River cutthroat trout throughout the native range of greenback cutthroat trout. The authors analyzed mitochondrial (COI, ND2) and nuclear (microsatellites, AFLP) DNA from 365 individuals from 15 locations in 3 major river drainage systems in Colorado and surrounding states. Distinct mtDNA lineages corresponding to each subspecies were corroborated by nuclear microsatellite and AFLP data. For another cautionary tale of repeated misidentification of a widely studied organism, see Siddall and colleagues’ entertaining June 2007 Proc R Soc paper scrutinizing commercially available medicinal leeches sold as Hirudo medicinalis.
Just as DNA analysis regularly overturns seemingly solid eyewitness identifications in crime investigations, routine DNA analysis can also help biologists avoid blunders. In 28 August 2007 Mol Ecol, researchers from University of Colorado, New Mexico State University, Pisces Molecular, and Brigham Young University report that over 20 years of restocking efforts in western US aimed at restoring native populations of endangered greenback cutthroat trout Oncorhynchus clarkii stomias have mostly been restocking a non-native, non-endangered subspecies, Colorado River cutthroat trout O. c. pleuriticus. They trace the confusion to repeated introductions beginning in the late 1800s of Colorado River cutthroat trout throughout the native range of greenback cutthroat trout. The authors analyzed mitochondrial (COI, ND2) and nuclear (microsatellites, AFLP) DNA from 365 individuals from 15 locations in 3 major river drainage systems in Colorado and surrounding states. Distinct mtDNA lineages corresponding to each subspecies were corroborated by nuclear microsatellite and AFLP data. For another cautionary tale of repeated misidentification of a widely studied organism, see Siddall and colleagues’ entertaining June 2007 Proc R Soc paper scrutinizing commercially available medicinal leeches sold as Hirudo medicinalis.
How might the future look with routine application of DNA ID as quality control? Incorporating DNA barcode analysis into Tree of Life studies is one useful approach, exemplified by two recent large-scale evolutionary studies published in January and April 2008 Syst Entomol, one on phylogenetic relationships in Saturnid silkmoths, and one on higher-level relationships among 12 families in ‘bombycoid complex’ of Lepidoptera. Both studies analyze COI barcodes of all specimens, “allowing confirmination of their identification for species present in the BOLD reference library and enabling future identifications of organisms whose identity is still pending.”
 On February 27, 2008,
On February 27, 2008, 
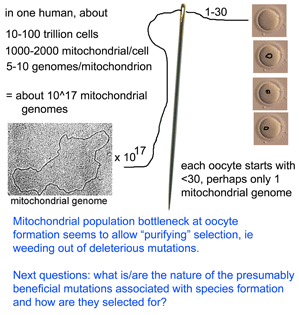 They then sequenced entire mitochondrial genomes from 190 of these progeny individuals in N2 to N6 generations (N2 is the first backcross that is homozygous normal at mtDNA mutator locus). To skip to the conclusion, most of the non-synonomous mitochondrial mutations were eliminated, leaving a pattern of excess synonymous mutations similar to that seen in human populations (which are the largest dataset so far for mitochondrial variation). The authors conclude that the mitochondrial population bottleneck known to occur at oogenesis, which deposits just one or few mitochondrial genomes per oocyte, means each mitochondrial genome must stand on its own so to speak, with the result that those eggs, embryos, or offspring harboring defective mitochondria will fail to survive. My figure at right tries to illustrate part of this process.
They then sequenced entire mitochondrial genomes from 190 of these progeny individuals in N2 to N6 generations (N2 is the first backcross that is homozygous normal at mtDNA mutator locus). To skip to the conclusion, most of the non-synonomous mitochondrial mutations were eliminated, leaving a pattern of excess synonymous mutations similar to that seen in human populations (which are the largest dataset so far for mitochondrial variation). The authors conclude that the mitochondrial population bottleneck known to occur at oogenesis, which deposits just one or few mitochondrial genomes per oocyte, means each mitochondrial genome must stand on its own so to speak, with the result that those eggs, embryos, or offspring harboring defective mitochondria will fail to survive. My figure at right tries to illustrate part of this process. 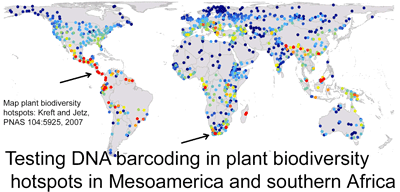
 mtDNA differences among these bivalves are remarkably large, even among species in the same genus. The differences among congeneric species in this sample (average 22%, range 12-42%) are larger than differences among entire class Aves (according to my analysis with BOLD software, COI differences among birds in different orders, such as penguins and hummingbirds for example, average 20%, with range 14-28%).
mtDNA differences among these bivalves are remarkably large, even among species in the same genus. The differences among congeneric species in this sample (average 22%, range 12-42%) are larger than differences among entire class Aves (according to my analysis with BOLD software, COI differences among birds in different orders, such as penguins and hummingbirds for example, average 20%, with range 14-28%).