We post “Future Knowledge of Life in Oceans Past”, the published version of Jesse’s opening speech to the October 2005 Census of Marine Life conference on Oceans Past (see What’s New 25 October 2005). Congratulations to David Starkey, Poul Holm, and Michaela Barnard and the History of Marine Animal Populations community on completing the book (Oceans Past: Management Insights from the History of Marine Animal Populations, D. J. Starkey, P. Holm, and M. Barnard, eds., Earthscan, London and Sterling VA, 2008).
News
Telescopes and DNA Barcodes
Cold Spring Harbor Laboratory’s Banbury Conference Center hosted the pair of meetings in 2003 that gave birth to the DNA barcoding movement. Jesse opened the Banbury meeting 28 October 2007 on Using Barcode Data in Studies of Molecular and Evolutionary Dynamics with a talk on Telescopes, Microscopes, Macroscopes and DNA Barcodes.
New York Times “dot earth” Poppies
New York Times environment reporter Andrew Revkin has launched a new “dot earth” blot with an entry about population that draws on the work of the Program for the Human Environment about implosion and explosion published in the journal article “Human Population Dynamics Revisited with the Logistic Model: How Much Can Be Modeled and Predicted?”
Abstract
Decrease or growth of population comes from the interplay of death and birth (and locally, migration). We revive the logistic model, which was tested and found wanting in early-20th-century studies of aggregate human populations, and apply it instead to life expectancy (death) and fertility (birth), the key factors totaling population. For death, once an individual has legally entered society, the logistic portrays the situation crisply. Human life expectancy is reaching the culmination of a two-hundred year-process that forestalls death until about 80 for men and the mid-80’s for women. No breakthroughs in longevity are in sight unless genetic engineering comes to help. For birth, the logistic covers quantitatively its actual morphology. However, because we have not been able to model this essential parameter in a predictive way over long periods, we cannot say whether the future of human population is runaway growth or slow implosion. Thus, we revisit the logistic analysis of aggregate human numbers. From a niche point of view, resources are the limits to numbers, and access to resources depends on technologies. The logistic makes clear that for homo faber, the limits to numbers keep shifting. These moving edges may most confound forecasting the long-run size of humanity.
Non-invasive DNA recovery leaves tiny specimens intact
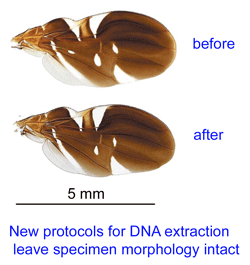 Reference databases of DNA sequences used for species identification, ie DNA barcode libraries, are most powerful when the morphologic specimens are vouchered in a museum collection. This way, when there are puzzling results, DNA and morphologic specimens can be re-examined. However to date it has been challenging to recover DNA from small organisms without destroying them in the process.
Reference databases of DNA sequences used for species identification, ie DNA barcode libraries, are most powerful when the morphologic specimens are vouchered in a museum collection. This way, when there are puzzling results, DNA and morphologic specimens can be re-examined. However to date it has been challenging to recover DNA from small organisms without destroying them in the process.
In Mol Ecol Notes 9 aug 2007 researchers from US Department Agriculture and Smithsonian Institution, National Museum of Natural History, describe a uniform protocol for “nondestructive extraction of DNA from terrestrial arthropods” including ticks, spiders, beetles, flies, and bees. 1 to 4 h in a guanidium thiocyanate extraction buffer yielded amplifiable COI DNA from most specimens. Inspection of specimens after extraction including with phase contrast and scanning electron microscopy demonstrated preservation of most morphologic characters.
In Mol Ecol Notes 27 june 2007, UK researchers (University College, London, NERC Centre for Ecology and Hydrology, Oxford, and UK Environmental Agency) describe a rapid, non-destructive, chemical-free method for DNA recovery from blackflies, including adult, larval, and pupal forms. Hunter et al report brief (1 minute) sonication in sterile water yielded 66% success with COI barcode amplification and preserved morphologic details.
These reports are exciting in the methods they describe and in how they highlight the general value of extracting DNA and determining DNA barcode sequences as an integral part of preparing traditional morphologic vouchers.
Wood H to C ratio
The current madness for biofuels has raised again the question of the proper hydrogen-to-carbon ratio to use to index wood and other biomass against other fuels. We offer a note on the proper H:C ratio for wood.
Neotropical birds: Argentine researchers speed past halfway point
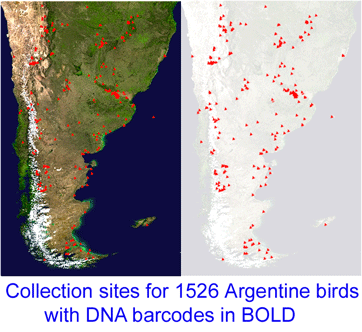
The Neotropics, comprising southern Mexico, Central America, Caribbean, and South America, is home to over 4,000 bird species, representing over 40% of world birds. In this post, Pablo Tubaro, Museo Argentino de Ciencias Naturales (MACN), Buenos Aires, Argentina, sends this update on DNA barcoding birds of Argentina:
 “This project, which started in December 2005, is a collaboration between MACN and the Biodiversity Insitute of Ontario/Canadian Center for DNA Barcoding (BIO/CCDB). In November 2006 the project was boosted by a grant from the Richard Lounsbery Foundation that supports expanded collecting efforts in Argentina, training of Argentine students at CCDB (2 trained so far), and establishment of a DNA laboratory at MACN.
“This project, which started in December 2005, is a collaboration between MACN and the Biodiversity Insitute of Ontario/Canadian Center for DNA Barcoding (BIO/CCDB). In November 2006 the project was boosted by a grant from the Richard Lounsbery Foundation that supports expanded collecting efforts in Argentina, training of Argentine students at CCDB (2 trained so far), and establishment of a DNA laboratory at MACN.
A special feature of this project is that it started literally from scratch. As there were no significant collections of frozen bird tissues with associated vouchers in Argentina, we started by resampling the country from north to south, conducting joint campaigns in collaboration with researchers from several North American institutions including American Museum of Natural History, Cornell University, Louisiana State University, Queen’s University, University of Alaska, and University of Kansas. At present our frozen tissue collection with associated vouchers includes more than 3100 samples and is growing rapidly. We will be doing field work at Iguazu National Park in November and December and aim to have collected 70% of Argentine birds by the year’s end.
Results so far show interspecific and intraspecific levels of divergence in COI squence are similar to published results with North American birds. In more than 98% of cases, the COI sequences belonging to different species do not overlap. In addition, in 3% of cases Argentine birds show distinct COI sequence clusters, suggesting the possible existence of cryptic species or geographical races that deserve species status. At this moment, four doctoral and post-doctoral fellowships have been requested or are already awarded by the National Research Council of Argentina (CONICET) and the National Science Foundation of Argentina (ANPCyT) to study in depth the phylogeographic structure of some of the interesting cases revealed by our DNA barcode survey.”
Congratulations to Pablo Tubaro and his team on their rapid progress in DNA barcoding Argentine birds, creation of a significant avian tissue and skin collection at MACN, and on recognition of the value of this work by science institutions in Argentina!
Lounsbery Political Attitudes
The Richard Lounsbery Foundation sponsored a new survey of political attitudes of American professors by Neil Gross (Harvard) and Solon Simmons (George Mason U.). Jesse offered opening remarks on behalf of Lounsbery at a lively symposium on “Professors and Their Politics” 6 October 2007 at Harvard to review and discuss the findings of the survey. Journalist Scott Jaschik provides and excellent account of the survey and symposium in Inside Higher Education.
CBOL Taipei
The movement to create a library of DNA barcodes for plants, animals, and fungi began with the Cold Spring Harbor Banbury meeting that Jesse Ausubel and Mark Stoeckle helped organize in 2003. During the 17-21 September 2007 the Consortium for the Barcode of Life convened its 2nd International Conference in Taipei along with meetings of working groups concerned with fish, all forms of marine life, plants, fungi, regional initiatives, and techniques. The progress, reported in a press release and covered in The Economist and also recounted in Mark’s Blog, is thrilling. The happy mood of the exciting conference shows in the photo of attendees. Thanks to Kwang-Tsao Shao (Academia Sinica), David Schindel (Consortium for the Barcode of Life), Karen Armstrong (New Zealand, chair of conference program committee), and many others for making a great success.
Taxonomy without borders
 341 researchers from 44 countries gathered for the Second International Barcode of Life Conference, held at Academia Sinica, Tapei, Taiwan on 17-21 September 2007 (program, participants, and abstracts at www.dnabarcodes2007.org).
341 researchers from 44 countries gathered for the Second International Barcode of Life Conference, held at Academia Sinica, Tapei, Taiwan on 17-21 September 2007 (program, participants, and abstracts at www.dnabarcodes2007.org).
Conference presentations highlighted a thrilling array of progress on diverse scientific and practical fronts since the First International Barcode of Life at The Natural History Museum, London, in February 2005 (London Conference proceedings in themed issue Phil Trans R Soc 360: 2005 available through Consortium for Barcode of Life (CBOL) website. I found the Tapei conference to be a landmark demonstration of the value to society and science of a standardized, inexpensive approach to identifying species through DNA, ie DNA barcoding. The Economist’s 20 September 2007 piece “Name, rank, and serial number” recaps results so far and looks ahead to near future societal benefits.
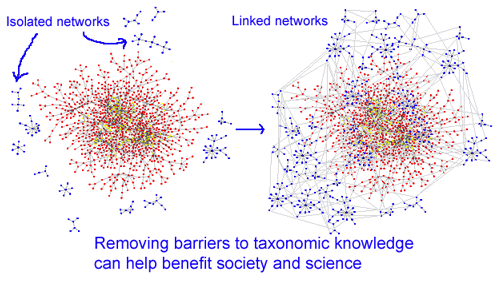
Near the close of the conference, David Schindel, Executive Secretary for CBOL, referred to the DNA barcode initiative as “taxonomy without borders”. Just as removing security fences benefits African wildlife, standardized inexpensive technology for species identification, ie DNA barcoding, is helping remove barriers that balkanize taxonomy and limit public access to biological knowlege. The DNA barcode initiative, together with the Encyclopedia of Life which includes digitizing the world’s taxonomic literature are creating powerful new ways of seeing biodiversity, with benefits to society and science.
I look forward to a future in which the multiple sectors of taxonomic and biodiversity science are densely linked to each other and public users.
Werewolf Microbes
“Werewolf Microbes might take power!” The e-zine Energy Tribune interviewed Jesse for its subscribers. We post the entire interview conducted by Robert Bryce.