Are USA carbon dioxide emissions now around their “natural†peak?  Following up on our postings 589 and 585 on 20 April 2009, we post a trio of figures that suggest that the energy system is decarbonizing as expected and in spite of the buzzing of pundits and politicians and policymakers, who remind us of the fly in the classic fable of Jean de la Fontaine (1621-1695), the Coach and the Fly.
The Coach and The Fly
Jean de la Fontaine (translation from French by Jesse Ausubel)
On a climbing, bad, sandy road,
Exposed to the sun on all sides
Six strong horses pulled a coach.
Women, a monk, old people had gotten out.
The team of animals sweated, panted, was spent.
A fly turned up, and approached the horses;
And pretends to excite them by its buzzing.
Stings one, stings another, and thinks at this moment
That she makes the machine go.
She sits on the shaft, on the nose of the coachman;
As soon as the coach moved,
And she saw the people walk,
She attributes uniquely to her herself all the glory;
Go, come, hurry; it seems the fly could be
A battle sergeant going to each spot
Making his troops advance, and hasten victory.
She cries she acts alone, and she has all the worries;
That no one helps the horses to pull out of this mess.
The Monk recites his breviary;
He takes his time! A woman sings;
As if this is a question of singing songs!
Lady Fly goes to buzz in their ears,
And does a hundred equally silly things.
After much work the coach arrives on top.
Let’s breathe now, says the fly immediately:
I have done so much that our people have finally reached the plateau.
So, my good horses, pay me for my pain.
Thus, certain people, eager to impress,
Introduce themselves into affairs:
They play the busybody everywhere,
And, everywhere importuning, they must be chased off.
 The horse-chestnut leaf miner moth Cameraria ohridella (link to
The horse-chestnut leaf miner moth Cameraria ohridella (link to 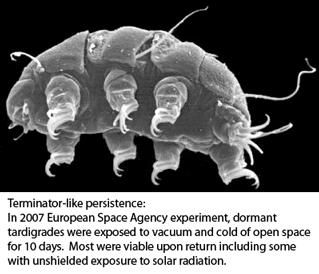 Tardigrades, commonly called water bears, are tiny (0.1-1.5 mm) water-dwelling invertebrates found in diverse environments. About 1000 species are known. Morphologic identification is difficult and may be limited to certain life stages–some species can be identified only from eggs, for example. Tardigrades can transform into a dormant state with remarkable ability to withstand extreme drying, cold, and radiation for prolonged periods, making them of interest for persons studying biology of tissue repair, aging and other fields.
Tardigrades, commonly called water bears, are tiny (0.1-1.5 mm) water-dwelling invertebrates found in diverse environments. About 1000 species are known. Morphologic identification is difficult and may be limited to certain life stages–some species can be identified only from eggs, for example. Tardigrades can transform into a dormant state with remarkable ability to withstand extreme drying, cold, and radiation for prolonged periods, making them of interest for persons studying biology of tissue repair, aging and other fields. “DNA barcoding involves sequencing a standard region of DNA as a tool for species identification. However, there has been no agreement on which region(s) should be used for barcoding land plants. To provide a community recommendation on a standard plant barcode, we have compared the performance of 7 leading candidate plastid DNA regions (atpF–atpH spacer, matK gene, rbcL gene, rpoB gene, rpoC1 gene, psbK–psbI spacer, and trnH–psbA spacer). Based on assessments of recoverability, sequence quality, and levels of species discrimination, we recommend the 2-locus combination of rbcL and matK as the plant barcode. This core 2-locus barcode will provide a universal framework for the routine use of DNA sequence data to identify specimens and contribute toward the discovery of overlooked species of land plants.”
“DNA barcoding involves sequencing a standard region of DNA as a tool for species identification. However, there has been no agreement on which region(s) should be used for barcoding land plants. To provide a community recommendation on a standard plant barcode, we have compared the performance of 7 leading candidate plastid DNA regions (atpF–atpH spacer, matK gene, rbcL gene, rpoB gene, rpoC1 gene, psbK–psbI spacer, and trnH–psbA spacer). Based on assessments of recoverability, sequence quality, and levels of species discrimination, we recommend the 2-locus combination of rbcL and matK as the plant barcode. This core 2-locus barcode will provide a universal framework for the routine use of DNA sequence data to identify specimens and contribute toward the discovery of overlooked species of land plants.”