Infectious diseases may determine survival of individuals, entire species, and perhaps even large branches on the Tree of Life. Beginning in the late 1970’s, rapid declines in amphibian populations around the globe were noted and today about 40% of world’s 6,671 amphibian species are threatened with extinction (e.g. Stuart et al 2004). The major cause appears to global dissemination of a pathogenic chytrid fungus, Batrachochytrium dendrobatidis, first reported in 1998 and formally described in 1999.
Although the global pattern is clear, many local population declines remain enigmatic due to absence of histologic data. In addition, the pattern of spread of the fungus and its timing in relation to mortality are not known. In April 2011 Proc Natl Acad Sci USA (open access), researchers from San Francisco State University and University of California, Berkeley, describe a non-invasive, DNA-based method for detecting B. dendrobatidis (Bd) in formalin-preserved specimens. Although exceptions are reported, DNA recovery after formalin treatment usually fails, so these are remarkable results.
Cheng and colleagues analyzed formalin-preserved salamander and frog specimens collected in Mexico, Guatemala, and Costa Rica in areas where population declines had occurred. Specimens were rinsed in 70% ethanol, then, using a skin swab or dental brush, “stroked 30 times over the ventral surface…from neck to vent” [salamanders] or “on the ventral surface, including the inner thighs, abdomen, and between toes” [frogs]; the swab/brush was then stored in a microfuge tube at 4 oC until processing. DNA was extracted with a standard kit (Prepman Ultra or Qiagen DNeasy), and a 146-bp segment of Bd ITS-1 region was amplified, using 1/80th of recovered DNA for each amplification, run in triplicate using real-time PCR along with positive and negative controls.
Initial trials were done with 29 Bd-infected (as determined by histology) and 9 Bd-uninfected formalin-preserved Batrochoseps salamander specimens. Bd was detected in in 24 (84%) of infected specimens and none of uninfected specimens. They suggest that their success with such unlikely specimens may reflect “(i) the very short length (146 bp) of the target sequence for Bd amplification, (ii) the presence of many copies per Bd cell of the ITS-1 region being targeted in our assay, and (iii) recovery of many cells of Bd in our swabbing technique because Bd grows on the skin surface of the host.”
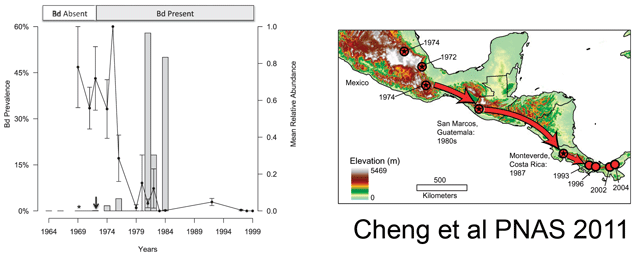
The researchers then applied this assay to frogs and salamanders collected in Mexico (n=537), Costa Rica (n=74), and Guatemala (n=615) between 1964 and 2010. They found Bd as early as 1972, with a large increase (>50% prevalence) beginning in 1980, coincident with the observed population declines (see figure above). Combining their results with those of Lips et al 2006 indicated a steady southward movement of Bd from southern Mexico in 1972 to Panama in 2004. They interpret this remarkably slow expansion to mean that the pathogen is spread by the animals themselves, perhaps as they move between the tiny pools of water that collect in the crowns of bromeliads. The near coincident appearance of Bd around the world suggests additional modes of spread, possibly including human activities. I look forward to additional studies that will shed light on the global dissemination of Bd and point to interventions to limit this ongoing disaster for amphibians.