In “Estimating diversity of Indo-Pacific coral reef stomatopods through DNA barcoding of stomatopod larvae” (FirstCite early online publication in Proceedings Royal Society Biology) Paul Barber and Sarah Boyce, Boston University, look at 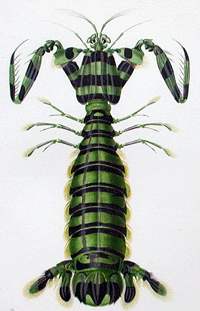 a what is thought to be a well-understood group, stomatopods, commonly known as mantis shrimp. Stomatopods are marine crustaceans distinct from true shrimp and are thought to include about 400 species worldwide. Like many marine species, they have a bipartite life cycle where dispersal is achieved through a planktonic larval developmental stage. However, larval stages are notoriously difficult to identify morphologically. Barber and Boyce first established a database of COI DNA barcodes from adult forms of nearly all known species. They then applied DNA barcoding to planktonic larvae collected in light traps at locations in the Pacific Ocean and Red Sea. Comparison of
a what is thought to be a well-understood group, stomatopods, commonly known as mantis shrimp. Stomatopods are marine crustaceans distinct from true shrimp and are thought to include about 400 species worldwide. Like many marine species, they have a bipartite life cycle where dispersal is achieved through a planktonic larval developmental stage. However, larval stages are notoriously difficult to identify morphologically. Barber and Boyce first established a database of COI DNA barcodes from adult forms of nearly all known species. They then applied DNA barcoding to planktonic larvae collected in light traps at locations in the Pacific Ocean and Red Sea. Comparison of  sequences from 189 larval forms revealed 22 distinct larval operational taxonomic units (OTUs), but a minority of these matched known species, suggesting that stomatopod species diversity is underestimated by a minimum of 50% to more than 150%. Their results support general use of DNA barcoding as a rapid, relatively-inexpensive first step in cataloging marine species with planktonic larvae. A similar approach has been applied on land by Smith et al “DNA barcoding for effective biodiversity assessment of a hyperdiverse arthropod group: the ants of Madagascar“.
sequences from 189 larval forms revealed 22 distinct larval operational taxonomic units (OTUs), but a minority of these matched known species, suggesting that stomatopod species diversity is underestimated by a minimum of 50% to more than 150%. Their results support general use of DNA barcoding as a rapid, relatively-inexpensive first step in cataloging marine species with planktonic larvae. A similar approach has been applied on land by Smith et al “DNA barcoding for effective biodiversity assessment of a hyperdiverse arthropod group: the ants of Madagascar“.
DNA barcode OTUs, such as found in these studies, are not equivalent to species descriptions and are not sufficient to establish systematic phylogeny. In my view, these studies indicate that DNA barcodes can be permanent indexers for filing and retrieving biologic information in the encyclopedia of life. Routinely incorporating DNA barcoding into biological surveys will enhance the long-term value of expensive field work.
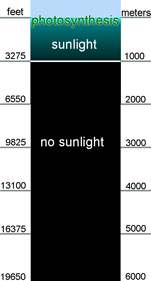 The deep oceans are the largest biotic space on earth, but remain largely unexplored.
The deep oceans are the largest biotic space on earth, but remain largely unexplored.  According to Ann Bucklin, lead scientist for CMarZ and Head of the University of Connecticut Marine Sciences Department, “we are just starting to realize how little we know about species variety. We used to think we knew many species well, but the advent of DNA barcoding has radically altered that perception. Genetically distinctive species of zooplankton are being found with increasing frequency.”
According to Ann Bucklin, lead scientist for CMarZ and Head of the University of Connecticut Marine Sciences Department, “we are just starting to realize how little we know about species variety. We used to think we knew many species well, but the advent of DNA barcoding has radically altered that perception. Genetically distinctive species of zooplankton are being found with increasing frequency.”  ABI’s smallest sequencer is about the size and weight of a house air conditioning unit [ABI 310: 95 kg (208 lbs); 61 x 56 x 86 cm (24 x 22 x 34 in)]. Researchers who developed the
ABI’s smallest sequencer is about the size and weight of a house air conditioning unit [ABI 310: 95 kg (208 lbs); 61 x 56 x 86 cm (24 x 22 x 34 in)]. Researchers who developed the 

 museum’s organizational and research involvement in the international scientific effort “to develop a system for rapidly and inexpensively identifying the approximately 1.7 million known flora and fauna species, and creating an electronic database for the estimated 10 million species across the planet”. As outlined by Cristian Samper, Museum Director, “the use of DNA barcoding in identifying and distinguishing species could revolutionize the way we do science”. The article concludes with an observation from Lee Weigt, Manager of the Museum’s Laboratories of Analytical Biology “What the human genome research can do for medicine, DNA barcoding can do for biology”
museum’s organizational and research involvement in the international scientific effort “to develop a system for rapidly and inexpensively identifying the approximately 1.7 million known flora and fauna species, and creating an electronic database for the estimated 10 million species across the planet”. As outlined by Cristian Samper, Museum Director, “the use of DNA barcoding in identifying and distinguishing species could revolutionize the way we do science”. The article concludes with an observation from Lee Weigt, Manager of the Museum’s Laboratories of Analytical Biology “What the human genome research can do for medicine, DNA barcoding can do for biology” it was not possible to identify the exact species, in this case whether this was an Allen’s (S. sasin) or Rufous (S. rufus), species native to the western U.S. that normally winter in Mexico. Even in the hand, identification can be difficult and in
it was not possible to identify the exact species, in this case whether this was an Allen’s (S. sasin) or Rufous (S. rufus), species native to the western U.S. that normally winter in Mexico. Even in the hand, identification can be difficult and in  spotted beneath the feeder was brought to University of Guelph, Ontario. DNA extracted from the feather and analyzed for COI barcode proved a match for S. rufus.
spotted beneath the feeder was brought to University of Guelph, Ontario. DNA extracted from the feather and analyzed for COI barcode proved a match for S. rufus.  ABBI Palearctic Regional Chair,
ABBI Palearctic Regional Chair,  Museum of Natural History. After study, Dr. Hoffman sent several specimens he could not identify to scientists in Italy. In July 2002, Italian scientists announced the Central Park centipede was a new species, and named it Nannarrup hoffmani in honor of Dr. Hoffman. In October 2003, Foddai, Bonato, Pereira, and Minelli published the species description in
Museum of Natural History. After study, Dr. Hoffman sent several specimens he could not identify to scientists in Italy. In July 2002, Italian scientists announced the Central Park centipede was a new species, and named it Nannarrup hoffmani in honor of Dr. Hoffman. In October 2003, Foddai, Bonato, Pereira, and Minelli published the species description in  filter-feeding whale shark Rhincodon typus.
filter-feeding whale shark Rhincodon typus.  in most parts of the world.” In The USA regulations prohibit possession of any part of protected species, but how to identify dried fins?
in most parts of the world.” In The USA regulations prohibit possession of any part of protected species, but how to identify dried fins?