The Program for the Human Environment bids a fond farewell to research assistant Smriti Rao, who worked with us beginning in October 2008 and now relocates to San Diego. We trust Smriti will remain part of the extended PHE family.
News
Census of Marine Life goes Experimental and Weird
The Census of Marine Creature video, produced by the CoML + National Geographic about the work of the deep sea teams of the CoML, has been nominated for a Webby, 8 June 2010 selection. The Webby Award is the leading international award honoring excellence on the Internet. The video features several species found by the Census of Marine Life in the deep sea, including a Piglet Squid and Football Octopod. Webby’s are awarded both by an expert jury and by popular polling. To vote for the video, visit https://www.youtube/webby and click “Experimental & Weird”, The Census of Marine Life video is last on the page. Support the Census of Marine Life by voting today.
Marine Life Around Martha’s Vineyard
The May-June 2010 issue of Martha’s Vineyard Magazine publishes “From cod to conch: How the fisheries have shifted focus over the past twenty-five years,†by Mike Secombe, with quotations from Jesse. https://www.mvmagazine.com/article.php?25218
The Hidden Majority of Marine Life
For a lively summary of the latest news from the Census of Marine Life, including still images and videos, visit the news release about “hard-to-see” creatures. Learn about a carpet of bacteria the size of Greece and 35 elephants of marine microbes for every human.
Buddhists & Marine Biodiversity
A Buddhist quarterly magazine publishes a short article by Jesse about the Census of Marine Life, “Census of Ocean Life: On the Difficulty and Joy of Seeing What Is Near and Far.”
Leishmaniasis: DNA helps ID vectors, parasite, control agent
Leishmaniasis is a chronic parasitic infection caused by various Leishmania species, kinetoplast protozoans related to Trypanosoma (the latter includes agents of African sleeping sickness and Chagas disease, suggested as a cause of Charles Darwin’s ill health in late life). Depending on the species involved, leishmaniasis manifests as illness ranging from non-healing cutaneous or mouth ulcers (CL) to sometimes fatal visceral infection (VL). In the Neotropics, 12 species infecting humans have been identified, all associated with CL. Neotropical leishmaniasis is mostly zoonotic (ie originates from animal reservoirs as opposed to human-to-human transmission), and the vectors are tiny phlebotomine sand flies, particularly Lutzomyia sp.
 In March 2010 PloS Neglected Trop Diseases investigators from Smithsonian Tropical Research Institute (STRI) and Instituto Conmemorativo Gorgas de Estúdios para la Salud, Panamá, apply DNA testing to Lutzomyia sandflies collected on Barro Colorado Island, STRI’s island home in the Panama Canal. Aiming to analyze as many species as possible, Azpurua and colleagues selected 435 individuals, which they morphologically identified as representing 16 Lutzomyia and 2 Brumptomyia sandfly species, for further analysis. Over 95% of specimens in the original collection were from one species, L. panamensis, so this was not a completely representative sample; nonetheless, “the relative abundances of species collected in this study were significantly correlated to those found in a previous intensive study of sand fly community composition on the [Panama] mainland…that collected over 30,000 Lutzomyia individuals in 35 species.”
In March 2010 PloS Neglected Trop Diseases investigators from Smithsonian Tropical Research Institute (STRI) and Instituto Conmemorativo Gorgas de Estúdios para la Salud, Panamá, apply DNA testing to Lutzomyia sandflies collected on Barro Colorado Island, STRI’s island home in the Panama Canal. Aiming to analyze as many species as possible, Azpurua and colleagues selected 435 individuals, which they morphologically identified as representing 16 Lutzomyia and 2 Brumptomyia sandfly species, for further analysis. Over 95% of specimens in the original collection were from one species, L. panamensis, so this was not a completely representative sample; nonetheless, “the relative abundances of species collected in this study were significantly correlated to those found in a previous intensive study of sand fly community composition on the [Panama] mainland…that collected over 30,000 Lutzomyia individuals in 35 species.”
To skip to the end, COI barcodes unambiguously assigned all 49 individuals to 18 distinct lineages corresponding to named species, plus highlighted 2 genetically-divergent individuals that might represent cryptic species. Using primers for ITS-1 (a nuclear gene) and mini-circle DNA (part of mitochondrial genome), Leishmania were detected in 2 of 5 human-biting species, Lu. trapidoi (13/30 individuals tested, 43.3%) and Lu. gomezi (5/19 individuals tested, 26.3%). By my estimate, taking into account relative abundances of Lutzomyia sp., about 1% of Barro Colorado Island sand flies carry Leishmania. Surprisingly, DNA sequencing identified the parasite as Le. naiffi, a South American species not previously reported in Panama. Finally, using the same set of DNA extracts, the researchers tested for Wolbachia, a rickettsial intracellular insect parasite and candidate biological control agent. Wolbachia were found in 3 of 18 species, including 50% of Lu. trapidoi, the main vector of CL in Panama. As an aside, I note that the presence of Wolbachia apparently did not interfere with discriminating among sand fly species; hypothesized interference from Wolbachia was one of the early worries some expressed about DNA barcoding (e.g Whitworth Proc Biol Sci 2007).
Standardized DNA testing enables many more persons to identify insects, regardless of life stage, including those that serve as vectors for human diseases. In this report by Azpurua and colleagues, the discovery of a new species of Leishmania for Panama, and possible undescribed Lutzomyia vectors, suggests that wide application of standardized DNA testing will lead to further discoveries relevant to control of human and animal infectious diseases.
Simplified DNA barcode recipe-skip step one
It used to be standard practice to shave the area around the incision before surgery, as it was thought that hair harbored bacteria that would cause wound infection. Beginning in the 1970s, doctors found this was unnecessary, and in fact was associated with higher incidence of post-operative infection. This history comes to mind in reading March 2010 BioTechniques report by researchers from University of Guelph demonstrating that DNA suitable for PCR and sequencing can be obtained simply by leaving specimens in alcohol overnight!
 Of the three steps required to get from a specimen to a DNA barcode, namely DNA isolation, PCR (polymerase chain reaction), and sequencing, the first step is the most labor intensive and hardest to automate. Numerous protocols/kits have been developed to optimize DNA isolation from various types of specimens, such as plant vs animal tissues. As described by the Guelph researchers, “these procedures force cells to release their DNA via physical pertubation and/or chemical treatment, which is then followed by a clean-up procedure in which unwanted cellular compoents are separated from the DNA.” The researchers “hypothesized that a small amount of DNA leaks from the tissue into the preservation solution (usually ethanol), and that this DNA was amplifiable using a standard PCR protocol.” To start, they analyzed Monte Alban mescal, which is sold with a “worm” (a caterpillar of the agave moth, Hypopta agavis) in each bottle. They evaporated 50 mL mescal, re-dissolved the residue in water, applied this to a Qiagen MinElute spin column, resuspended the product in 50 ?L water, and used 2 ?L of resulting solution in a standard 25 ?L PCR reaction, with successful amplification and sequencing of 130 base mini-barcode of COI. This case was presumably challenging as mescal is only 40% ethanol and contains a variety of material that might inhibit PCR. In subsequent tests, 1 mL of 95% ethanol used to preserve specimens was evaporated, resuspended in 30 ?L of water without column purification, and 2 ?L used for PCR.
Of the three steps required to get from a specimen to a DNA barcode, namely DNA isolation, PCR (polymerase chain reaction), and sequencing, the first step is the most labor intensive and hardest to automate. Numerous protocols/kits have been developed to optimize DNA isolation from various types of specimens, such as plant vs animal tissues. As described by the Guelph researchers, “these procedures force cells to release their DNA via physical pertubation and/or chemical treatment, which is then followed by a clean-up procedure in which unwanted cellular compoents are separated from the DNA.” The researchers “hypothesized that a small amount of DNA leaks from the tissue into the preservation solution (usually ethanol), and that this DNA was amplifiable using a standard PCR protocol.” To start, they analyzed Monte Alban mescal, which is sold with a “worm” (a caterpillar of the agave moth, Hypopta agavis) in each bottle. They evaporated 50 mL mescal, re-dissolved the residue in water, applied this to a Qiagen MinElute spin column, resuspended the product in 50 ?L water, and used 2 ?L of resulting solution in a standard 25 ?L PCR reaction, with successful amplification and sequencing of 130 base mini-barcode of COI. This case was presumably challenging as mescal is only 40% ethanol and contains a variety of material that might inhibit PCR. In subsequent tests, 1 mL of 95% ethanol used to preserve specimens was evaporated, resuspended in 30 ?L of water without column purification, and 2 ?L used for PCR.
By evaporating 1 mL of ethanol in which specimens had been stored overnight (out of 2 mL total ethanol volume) and re-suspending residue in water, Shokralla and colleagues amplified and sequenced 130-base and 650-base fragments of COI and 1100-base fragment of 28s RNA from 25 whole insect specimens (mayflies, caddisflies; 1 gave COI only) and rbcL from 45 plant specimens (0.5 mm leaf samples). They also obtained COI sequences by sampling 1 mL of ethanol solution from 7 insect specimens stored at room temperature for 7 to 10 years. The researchers note this approach could facilitate for “high-throughput” analyses, as it involves liquid handling which is easy to automate, avoids destructive sampling, and could be used even when “there is simply no sample left for further analysis.” They conclude with a caution about “field sampling procedures that include placing mixtures of specimens in an ethanol jar” as this “may increase the chance of cross-contamination.”
The remarkably simple procedure reported by Shokralla and colleagues offers benefits to many persons who want to get DNA barcode identifications. I look forward to applications of this method in research and commercial laboratories, classrooms, and perhaps kitchens!
Update-Cardiovascular vs. Cancer
Using more recent data, we updated our analysis of Death and the Human Environment published in 2001 on competition between cancers and cardiovascular conditions as the leading cause of death in the US. We revisited the paper’s Figure 8, which projected cancers overtaking heart diseases about the year 2015.
In the past decade the fraction of deaths from cardiovascular conditions has decreased significantly, from 42% in 1993 to 34 % in 2006, as forecast. However, unexpectedly, the fraction of deaths resulting from malignant neoplasms has remained level (~23%). Fitting logistic curves to the updated dataset, we deduce that if the trends remain stable, the crossover is now likely deferred to about 2025.
Declining rates of smoking are likely a major contributer to the steady decline in cardiovascular deaths since 1965 and the more recent flattening of cancer-deaths. For example, epidemiologists associated with the American Cancer Society recently reported a 30% decline in age-standardardized cancer death rates since 1990, resulting “mostly from reductions in tobacco use, increased screening allowing early detection of several cancers, and modest to large improvements in treatment for specific cancers” (Jemal et al PLoS ONE, March 2010). The temporal trends in cardiovascular and cancer deaths may reflect differential effects of stopping smoking, as tobacco cessation has a large, relatively immediate benefit for cardiovascular disease, whereas reductions in cancer are more modest and delayed.
Food Fraud – Washington Post
Our high school DNA study with Brenda Tan and Matt Cost was the lead item in front-page article on “food fraud†in Washington Post on March 30, 2010. In addition to detailing the students’ findings, reporter Lyndsey Layton quotes PHE researcher Mark Stoeckle on DNA barcode testing of food, “If it’s simple enough that high school students with some supervision can do it, it moves out of the research application to something you can do regularly.† The WP article was widely reported and discussed in food-related blogs, and prompted a statement from Congressman John Dingell (MI), noting “more evidence of…insufficient authorities and resources to ensure the quality and safety of the U.S. food supply,†and calling for passage of H.R. 2749, the Food Safety Enhancement Actâ€.
For accurate census, birds await their barcodes
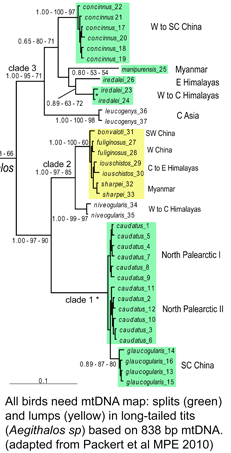 Although birds have been studied in more detail than any other large group of animals, mtDNA continues to reveal many overlooked species, such that named taxa turn out to be comprised of two or more distinct species. These revisions include some very familiar birds, e.g., Canada Goose, which was recently recognized as comprising two species, Cackling Goose (B. hutchinsii) and Canada Goose (B. canadensis) (A.O.U. Check-list 45th suppl. 2004); for a current example, see Päckert et al Mol Phylogenet Evol 2010). Although such taxonomic revisions reflect a combined analysis of morphological, behavioral (particularly song), geographic range, and DNA information, to my reading mtDNA generally trumps the other data, which mostly serve as corroborating evidence. It is not that mtDNA similarities or differences are important per se, it is that they are strongly predictive of the presence or absence of organismal differences, particularly reproductive isolation, that are the hallmarks of species status. Of the species examined so far (I estimate about 1/3 of the 10,000 world birds), most demonstrate a similar patterning of limited mtDNA differences within species and relatively large differences among species, so we can be confident that this analytic approach will hold up. As an aside, referring to splits of named species as “taxonomic inflation” is misleading, as it suggests the real number of species is already known, which seems no more correct than referring to binary stars resolved with a new telescope as reflecting “stellar inflation.”
Although birds have been studied in more detail than any other large group of animals, mtDNA continues to reveal many overlooked species, such that named taxa turn out to be comprised of two or more distinct species. These revisions include some very familiar birds, e.g., Canada Goose, which was recently recognized as comprising two species, Cackling Goose (B. hutchinsii) and Canada Goose (B. canadensis) (A.O.U. Check-list 45th suppl. 2004); for a current example, see Päckert et al Mol Phylogenet Evol 2010). Although such taxonomic revisions reflect a combined analysis of morphological, behavioral (particularly song), geographic range, and DNA information, to my reading mtDNA generally trumps the other data, which mostly serve as corroborating evidence. It is not that mtDNA similarities or differences are important per se, it is that they are strongly predictive of the presence or absence of organismal differences, particularly reproductive isolation, that are the hallmarks of species status. Of the species examined so far (I estimate about 1/3 of the 10,000 world birds), most demonstrate a similar patterning of limited mtDNA differences within species and relatively large differences among species, so we can be confident that this analytic approach will hold up. As an aside, referring to splits of named species as “taxonomic inflation” is misleading, as it suggests the real number of species is already known, which seems no more correct than referring to binary stars resolved with a new telescope as reflecting “stellar inflation.”
As with most animal groups, there are no nuclear genes that regularly distinguish closely-related birds. (It seems likely that sequencing entire nuclear genomes will enable discriminating species units, but this is inherently a more costly, less standardizable approach, making it unattractive for routine use.) Differences among closely-related species are more or less evenly-distributed throughout the mitochondrial genome, so that approximately 500-1000 bp of coding DNA usually contains sufficient information to recognize species and create genus-level phylogenies. Moreover, if species are not distinguished by this length of mtDNA then additional sequencing usually does not improve the resolution. This might be better stated the other way around, namely, if two sets of birds cannot be distinguished by a relatively short stretch of mitochondrial DNA, then they most likely belong to the same species. Why is this so? The prevailing null hypothesis is that mitochondrial differences are an accidental if useful accompaniment to species status, reflecting genetic drift over the several hundred thousand years of reproductive isolation presumably required for new species to emerge. In a recent essay (Nature 18 nov 2009), Nick Lane explores the interesting possibility that mitochondrial differences cause speciation, thus producing what they identify.
What barcoding adds to the historical approaches in avian systematics is standardization, so that the same locus is analyzed in every specimen, which speeds completion of species-level avian taxonomy and facilitates large-scale comparisons. Given that most bird species have yet to analyzed for species-level genetic differences, the standardized approach can save considerable time and money, as the results of independent investigators can be readily merged (e.g. Johnsen et al J Ornithol 2010, Kerr et al Frontiers Zool 2009). Beyond classification, this approach creates a reference library of avian barcodes, which enables identifying unknown specimens, such as from birdstrikes (Marra et al Frontiers Ecol Environ 2009). Our survey of avian frozen tissue collections identified over 315,000 specimens representing over 7,200 species (Stoeckle and Winker, Auk 2009). Based on what is in GenBank, it appears that most of these specimens have never been analyzed for any genetic locus, so a first-pass effort to sequence COI barcode region will certainly reveal many new species and help resolve higher-level relationships as well. There is an opportunity for a granting agency or foundation to have a large impact on avian systematics for modest cost by supporting barcoding of existing collections, given that the tissue specimens and vouchers, which are the most expensive components of collections, have already been prepared. Looking further ahead, such an effort would also create sets of high-quality DNA extracts, with species identity confirmed by COI barcode, linked to vouchered specimens, that would be a powerful resource for further genetic study.