Vast microbial genetic diversity found in oceans, stimulating new informatics tools
Tuesday, April 3rd, 2007
The biological universe is much larger and more diverse than we thought. In three papers in March 2007 PLoS Biology, scientists report on a genetic survey of microbial diversity in the world’s oceans. A large collaboration, the Global Oceanic Sampling (GOS), led by Craig Venter, analyzed microbial DNA collected by filtering seawater at 250 sites along a several thousand kilometer transect from the North Atlantic, through the Panama Canal, around the Galapagos Islands, ending in the Cocos Islands of the South Pacific. The resulting DNA dataset consisted of 6.3 billion base pairs (twice the size of the human genome), with 85% of the assembled and 57% of the unassembled data unique at a 98% identity cutoff. The extreme diversity prevented assembly of complete genomes, as many reads were unique. A comprehensive dataset of GOS sequences combined with pre-exisiting databases reveals nearly 6.12 million proteins, nearly doubling the number of known proteins. Some families of microbial proteins discovered in this study, particularly protein kinases, were previously thought to be restricted to eukaryotic organisms. Over 1700 sequence clusters show no identity to known families, implying we are far from knowing the full range of what proteins can do.
How to make sense of all this data? First, more data is needed!, namely more complete genomes into which the unassembled fragments can be placed. Second, new analytic tools. A new genomics and informatics group based at the California Institute for Telecommunications and Information Technology in San Diego, have built a metagenomics version of GenBank, known as the Community Cyberinfrastructure for Advanced Marine Microbial Ecology Research and Analysis (try saying that 3 times quickly!) which is fortunately known by acronym CAMERA.
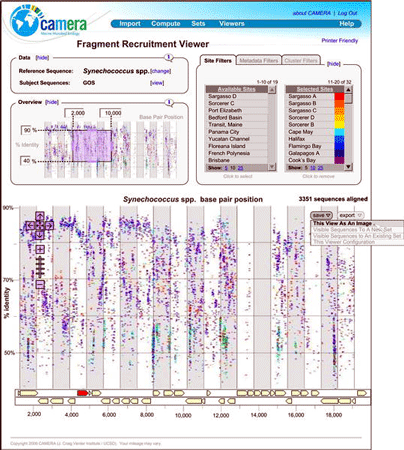
Just as Google and other search engines solved a problem of information overload that did not exist a few years ago, I am confident that CAMERA and other new informatics tools will enable us to view the expanding universe of environmental genomics, including DNA barcode libraries, in ways that will provide new understanding.
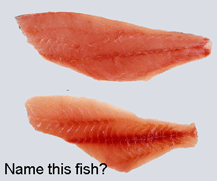
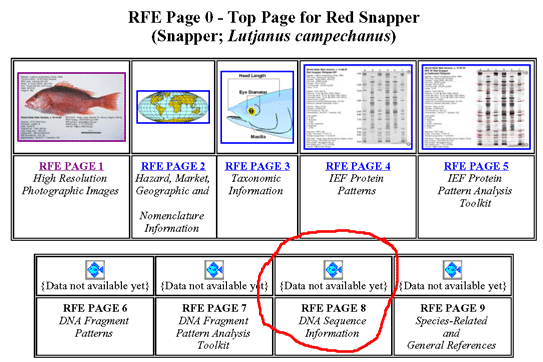
 A dozen articles in current issue of
A dozen articles in current issue of 
 The Indomalayan biogeographic region spans a vast area of tropical biodiversity and includes inumerable islands with high numbers of endemic species. A large scale genetic survey with DNA barcoding is likely to help lead to dramatic increases in species counts in particular and better understanding of biodiversity in general. Additional collecting may be particuarly important in this region, as it is at present the least well-represented in frozen tissue collections. There was strong enthusiasm among regional participants, and recognition the initiative has public appeal and the potential to engage new sources governmental support.
The Indomalayan biogeographic region spans a vast area of tropical biodiversity and includes inumerable islands with high numbers of endemic species. A large scale genetic survey with DNA barcoding is likely to help lead to dramatic increases in species counts in particular and better understanding of biodiversity in general. Additional collecting may be particuarly important in this region, as it is at present the least well-represented in frozen tissue collections. There was strong enthusiasm among regional participants, and recognition the initiative has public appeal and the potential to engage new sources governmental support. I look forward to organizational and scientific progress in this exciting region.
I look forward to organizational and scientific progress in this exciting region.  Two papers in early online
Two papers in early online 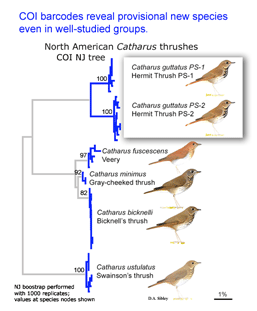 Birds being conspicuous, vocal, diurnal animals it is surprising that there are what appear to be overlooked species, even in an intensively-studied temperate region with relatively few species. Of course barcode clusters are not proof of species status, but to my knowledge all such divergent lineages either correspond to recognized species, or have subsequently been found to show biological covariants and have ultimately been granted species status.
Birds being conspicuous, vocal, diurnal animals it is surprising that there are what appear to be overlooked species, even in an intensively-studied temperate region with relatively few species. Of course barcode clusters are not proof of species status, but to my knowledge all such divergent lineages either correspond to recognized species, or have subsequently been found to show biological covariants and have ultimately been granted species status.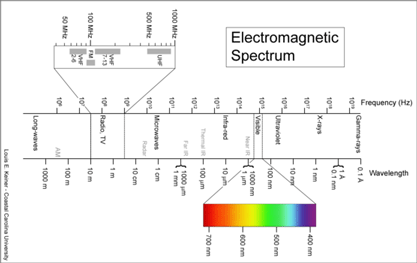 I see the “barcode map of genetic diversity” as analogous to an astronomical sky map that uses just a slice of the electromagnetic spectrum. It does not contain all the information necessary to understand the universe, but by focusing on one part of the spectrum it enables results from various studies to be seamlessly combined and allows both large and small scale comparisions.
I see the “barcode map of genetic diversity” as analogous to an astronomical sky map that uses just a slice of the electromagnetic spectrum. It does not contain all the information necessary to understand the universe, but by focusing on one part of the spectrum it enables results from various studies to be seamlessly combined and allows both large and small scale comparisions. 


 Rather than cracking the tough nut of an ideal plant barcode, Taberlet and co-authors look at a simple approach “emphasizing the point of view of scientists other than taxonomists“, and test this on food plants in archeological and industrial applications. The chloroplast trnL intron is not the most variable non-coding region in chloroplast DNA and does not differ enough to separate many closely-related plant species. On the plus side, there are robust primers which amplify the intron from diverse species. Like other group I introns, the trnL intron sequence has catalytic activity and a conserved secondary structure with alternating conserved and variable sequence domains. Taking advantage of this feature, the researchers designed primers to amplify one of the variable domains, the P6 loop. Binding sites for both the trnL primers, which amplify the entire intron, and the P6 loop primers are “highly conserved among land plants, from Angiosperms to Bryophytes“. Importantly, the P6 loop is only 10 to 143 bp and can be amplified from degraded DNA.
Rather than cracking the tough nut of an ideal plant barcode, Taberlet and co-authors look at a simple approach “emphasizing the point of view of scientists other than taxonomists“, and test this on food plants in archeological and industrial applications. The chloroplast trnL intron is not the most variable non-coding region in chloroplast DNA and does not differ enough to separate many closely-related plant species. On the plus side, there are robust primers which amplify the intron from diverse species. Like other group I introns, the trnL intron sequence has catalytic activity and a conserved secondary structure with alternating conserved and variable sequence domains. Taking advantage of this feature, the researchers designed primers to amplify one of the variable domains, the P6 loop. Binding sites for both the trnL primers, which amplify the entire intron, and the P6 loop primers are “highly conserved among land plants, from Angiosperms to Bryophytes“. Importantly, the P6 loop is only 10 to 143 bp and can be amplified from degraded DNA. In
In