CSI for fish: High school students help showcase ease of DNA-based species identification
Tuesday, August 26th, 2008
 In Pacific Fishing September 2008 (issue available on newstands, not yet on web) two New York City teenagers, Kate Stoeckle (my daughter!), 19, and classmate Louisa Strauss, 18, apply DNA-based identification to fish sold in their Manhattan neighborhood. The girls purchased 60 items from 14 establishments and sent samples to University of Guelph where graduate student Eugene Wong performed DNA barcode analysis. 14 (25%) of 56 samples with recoverable DNA were mislabeled, in all cases as more expensive or more desirable fish. Mislabeled items were sold at 2 of 4 restaurants and 6 of 10 grocery stores/fish markets.
In Pacific Fishing September 2008 (issue available on newstands, not yet on web) two New York City teenagers, Kate Stoeckle (my daughter!), 19, and classmate Louisa Strauss, 18, apply DNA-based identification to fish sold in their Manhattan neighborhood. The girls purchased 60 items from 14 establishments and sent samples to University of Guelph where graduate student Eugene Wong performed DNA barcode analysis. 14 (25%) of 56 samples with recoverable DNA were mislabeled, in all cases as more expensive or more desirable fish. Mislabeled items were sold at 2 of 4 restaurants and 6 of 10 grocery stores/fish markets.
The frequency of mislabeling and the ease of high-schoolers obtaining DNA-based species identifications captured public interest. Their study was featured on page 1 in New York Times on August 22, one of the “quotes of the day” on CNN/Time website, a live segment on CBS TV Early Show, an interview on national public radio, and has appeared in over 350 print and news items and blogs from 34 countries in 10 languages so far, with particularly heavy coverage in China, Korea, and Japan, presumably related to dietary importance of fish in general and sushi in particular.
This response demonstrates how powerful the FishBOL database is already (30,665 barcodes from 5,463 species so far, which represents about 20% of world fish), and hints at enormous uses that DNA barcoding will have as technology gets smaller and cheaper.

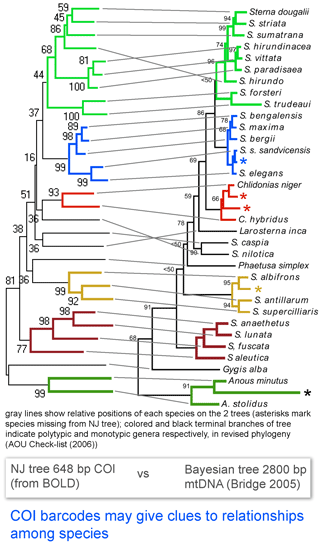 Here I look at one example from birds, comparing differences among COI barcodes to a recently revised phylogeny of terns (subfamily Sternini). According to
Here I look at one example from birds, comparing differences among COI barcodes to a recently revised phylogeny of terns (subfamily Sternini). According to  In
In  In
In 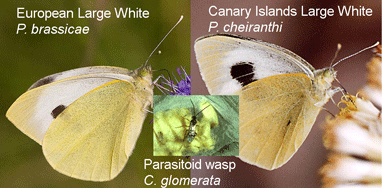 About 10% of named insect species are parasitoids, mostly wasps, but recognizing these often minute insects can be tricky. In
About 10% of named insect species are parasitoids, mostly wasps, but recognizing these often minute insects can be tricky. In  In
In  How many giraffes were onboard the Ark? Giraffes are classified as a single species, Giraffa camelopardalis, with five to nine subspecies proposed based on regional variation in pelage (coat pattern). In
How many giraffes were onboard the Ark? Giraffes are classified as a single species, Giraffa camelopardalis, with five to nine subspecies proposed based on regional variation in pelage (coat pattern). In 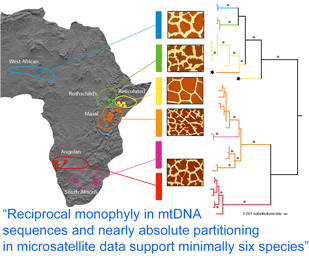 Analysis of 14 nuclear microsatellites from 381 individuals at 18 locations (it is not clear whether these are the same individuals as above) recovered the same six groups and suggested additional genetic subdivisions within some groups. Although at least some of the genetically and pelage-defined clusters have overlapping or adjacent ranges without geographic barriers, only three (0.8%) of individuals were identified as hybrids. These findings raise interesting questions about giraffe biology; for example, is there behavioral isolation perhaps based on visual recognition of pelage patterns?
Analysis of 14 nuclear microsatellites from 381 individuals at 18 locations (it is not clear whether these are the same individuals as above) recovered the same six groups and suggested additional genetic subdivisions within some groups. Although at least some of the genetically and pelage-defined clusters have overlapping or adjacent ranges without geographic barriers, only three (0.8%) of individuals were identified as hybrids. These findings raise interesting questions about giraffe biology; for example, is there behavioral isolation perhaps based on visual recognition of pelage patterns? 
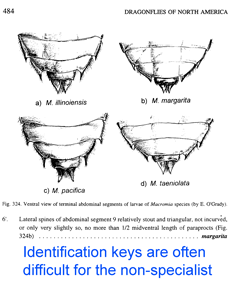 Just two years ago,
Just two years ago,  In addition to helping identify what is already known, DNA analysis can reveal what would otherwise remain hidden. In
In addition to helping identify what is already known, DNA analysis can reveal what would otherwise remain hidden. In